MEDIUM
Earn 100
In lattice, if a plane is drawn parallel to layers and , at a distance above the layer , it passes through centres of tetrahedral and octahedral voids. A plane parallel to layer , at a distance just below it passes through centres of tetrahedral and octahedral voids. Find . is the cell parameter of hcp lattice, the vertical edge length.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Solid State
HARD
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
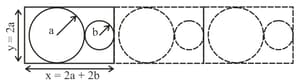
The ratio for which the packing fraction is minimized is closest to:
MEDIUM
EASY
HARD
EASY
EASY
MEDIUM
What are the examples of hexagonal close-packed structure?
HARD
EASY
EASY
EASY
MEDIUM
HARD
MEDIUM
The pattern in Hexagonal close packing and cubic close packing are same. Is the statement true or false?
HARD
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM

