MEDIUM
Earn 100
In nitroso-oxime tautomerism, the oxime form is more stable than the nitroso form.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Fundamentals of Organic Chemistry
HARD
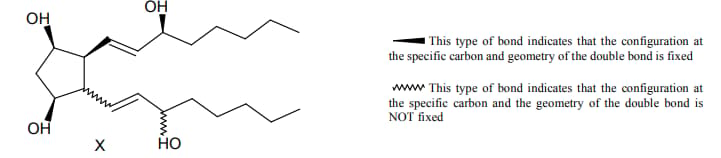
For the given compound X, the total number of optically active stereoisomers is ____.
EASY
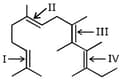
Geometrical isomerism is not possible at the site(s):
EASY
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
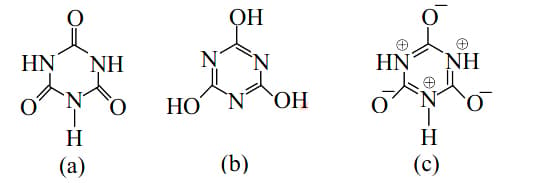
The tautomerism involving (1, 5) migration of hydrogen atom can be observed in
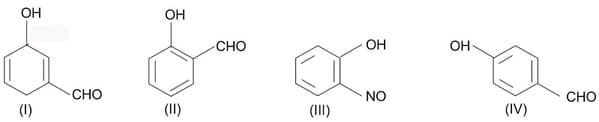
MEDIUM
Which of the two tautomers is more stable?
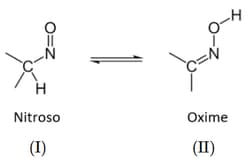
HARD
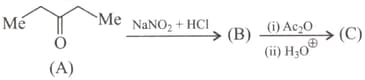
The final product is :
MEDIUM
EASY
MEDIUM
If is catalyst. What is the compound ?
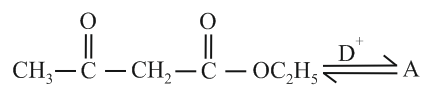
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and