EASY
Earn 100
In normal spinel structures the percentage of tetrahedral void occupied is
(a)
(b)
(c)
(d)None of these
50% studentsanswered this correctly
Important Questions on Develop models to describe the atomic composition of simple molecules and extended structures.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
HARD
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
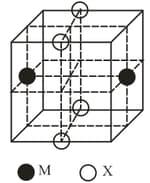
HARD
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
In the following circuit diagram, the current through the battery is
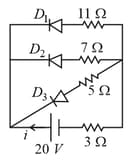
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
Match the following
List - I List-II
A) Metallic solid I) Carbon tetrachloride
B) Covalent solid II) Calcium fluoride
C) Molecular solid III) Copper
D) Ionic solid IV) Silicon carbide
V) Glass
The correct answer is
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
HARD
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
EASY
Physical Sciences>Matter and Its Interactions>Develop models to describe the atomic composition of simple molecules and extended structures.>Structure and Properties of Matter - Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.

