In period , element is to the right of the element . Then, find which of the elements have:
High electronegativity
Important Questions on Periodic classification of elements
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which element will be larger in size W or Z?
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
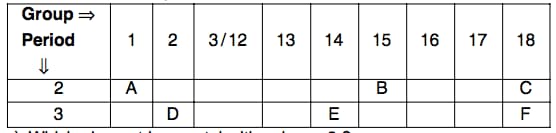
Out of D and E which one has a bigger atomic radius and why?
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which element belongs to the second period?
What is the basis of the classification of elements of the modern periodic table?
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which element represents an inert gas?
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
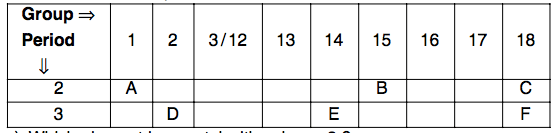
Which element is a metal with valency 2?
Give a reason for the following.
Ionisation potential increases across a period, from left to right.
Element forms a chloride with formula . Element would be most likely in the same group of periodic table as:
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
State whether A is a metal or non-metal.
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
Will C be larger or smaller in size than B.
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
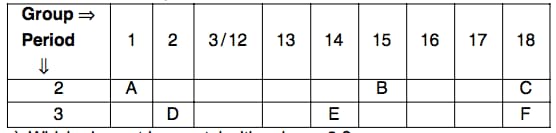
Which element is a non-metal with valency 3?

