EASY
CUET (UG)
IMPORTANT
Earn 100
In the Bohr's model of the hydrogen atom, the lowest orbit corresponds to
(a)infinite energy
(b)maximum energy
(c)minimum energy
(d)zero energy
50% studentsanswered this correctly

Important Questions on Atoms
EASY
CUET (UG)
IMPORTANT
A. The wavelengths of the spectral lines of the Lyman series are greater than the wavelength of the second spectral line of the Balmer series.
B. The orbits correspond to circular standing waves in which the circumference of the orbit equals a whole number of wavelengths.
EASY
CUET (UG)
IMPORTANT
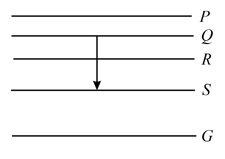
Figure shows the energy levels , , , and of an atom, where is the ground state. A red line in the emission spectrum of the atom can be obtained by an energy level change from to . A blue line can be obtained by the following energy level change
EASY
CUET (UG)
IMPORTANT
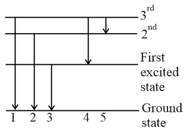
The figure indicates the energy level diagram of an atom and the origin of five spectral lines in emission spectra. Which of the spectral lines will also occur in the absorption spectra?
EASY
CUET (UG)
IMPORTANT
Consider the following two statements:
(A) Line spectra contain information about atoms.
(B) Band spectra contain information about molecules.
MEDIUM
CUET (UG)
IMPORTANT
EASY
CUET (UG)
IMPORTANT
EASY
CUET (UG)
IMPORTANT
EASY
CUET (UG)
IMPORTANT
