MEDIUM
Earn 100
In the Bohr's orbit, what is the ratio of total kinetic energy and the total energy of the electron?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Structure of Atom
MEDIUM
The difference between the radii of and orbits of is . The difference between the radii of and orbits of is Ratio of is :
EASY
HARD
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
(Planck's constant, mass of electron charge of electron permittivity of vacuum, )
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
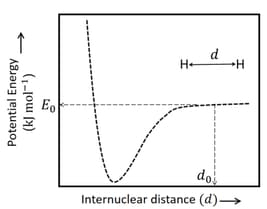
The figure below is the plot of potential energy versus internuclear distance of molecule in the electronic ground state. The value of the net potential energy (as indicated in the figure) is for at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent, find the value of to the nearest integer value.
As reference, the potential energy of atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as Give an answer to the nearest integer value.
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
EASY

