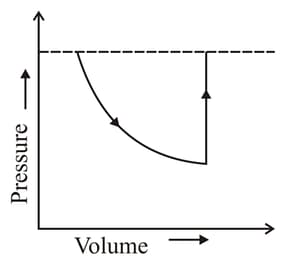
In the diagram shown in the figure, is a semicircle. Find the work done in the process .


Important Questions on Thermodynamics
A sample of an ideal gas has pressure , volume and temperature . It is isothermally expanded to twice its original volume. It is then compressed at constant pressure to have the original volume . Finally, the gas is heated at constant volume to get the original temperature.
(a) Show the process in a diagram.
(b) Calculate the total work done in the process.
Two moles of an ideal monatomic gas undergo a cyclic process as shown in the figure. The temperatures in different states are given as . Determine the work done by the gas during the cycle.
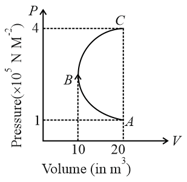
Two moles of helium gas are taken along the path (as shown in figure). Find the work done by the gas.
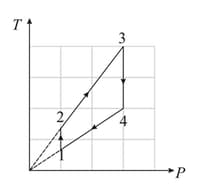
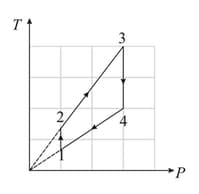
Two moles diatomic ideal gas are taken through the process const. Its temperature is increased from to . Find work done by the system?
A cylinder contains moles of oxygen at a temperature of . The cylinder is provided with a frictionless piston which maintains a constant pressure of on the gas. The gas is heated until its temperature rises to .
(a) How much work is done by the gas in the process?
(b) What is the change in the internal energy of the gas?
(c) How much heat was supplied to the gas?
In given figure, a sample of an ideal gas initially having internal energy is allowed to expand adiabatically performing work . Heat is then supplied to it, keeping the volume constant at its new value, until the pressure raised to its original value. The internal energy is then .