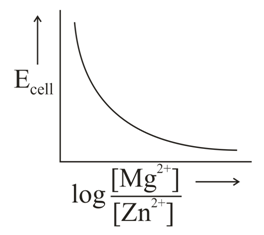
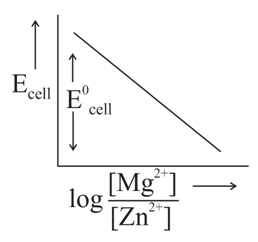
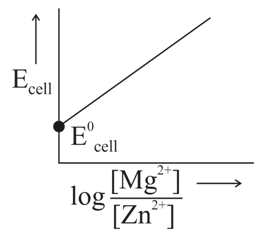
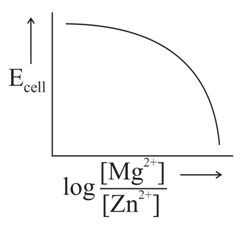
In the electrochemical cell,
The correct plot of versus will be represented as

Important Questions on Electrochemistry
Calculate the equilibrium constant for the following reaction at :
The standard EMF of the corresponding voltaic cell is .
Answer correct up to three places of decimals.
Calculate the equilibrium constant for the following reaction at :
The standard EMF of the corresponding voltaic cell is .
Answer correct up to two places of decimals with proper rounding off.
of of solution is electrolysed by a current of for .
How long will the same current have to pass through the solution to remove completely the metal ions from the solution?
Give the answer in seconds.
For the electrolytic production of from as per reaction:
How many faradays of electricity would be required to produce of ?
Molten is electrolysed with a current of to produce
How many gram equivalents of were produced?
Four different solutions containing each of are being electrolysed by using inert electrodes. In how many samples, metal ions would be deposited at cathode?
Given:
