In the following compressibility factor v/s pressure graph, which is true?
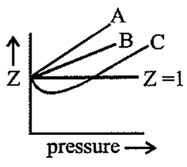

Important Questions on States of Matter: Gases and Liquids
A gas has a compressibility factor of and a molar volume of at a temperature of and pressure . If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be . The value of is _________
[Use: Gas constant, ]

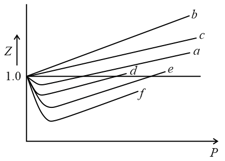
Among the gases and , the gases that show only positive deviation from ideal behavior at all pressures in the graph are
The isotherms of a gas are shown below :
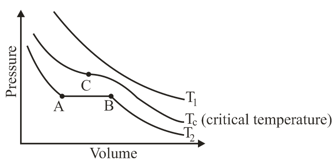
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :
The variation of the compressibility factorwith pressure for some gases, are shown in the figure below. Identify the gases and .
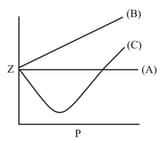
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
[Given : " a " and " b " are standard parameters for van der Waals' gas]

