MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
In the following diagram bunsen flame the represents.
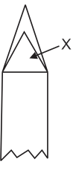

(a)Oxidising zone
(b)Reducing zone
(c)Lower temperature zone
(d)Hottest portion of flame
50% studentsanswered this correctly

Important Questions on Qualitative Analysis
MEDIUM
JEE Main/Advance
IMPORTANT
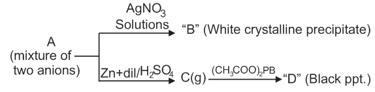
Then A may have:
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Anion of could be:
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
The acidic aqueous solution of Ferrous ion forms a brown complex in the presence of by the following two steps :
Complete and balance the equations.
