In the following reaction ,
.
where '' sign indicates rate of disappearance of the reactant. Thus, is

Important Questions on Chemical Kinetics
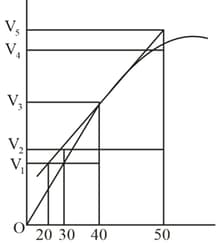
A graph of volume of hydrogen released vs time for the reaction between Zinc and dilute is given in figure. On the basis of this mark the correct statement from the following.
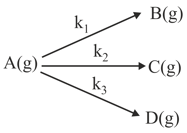
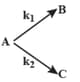
A gaseous compound A reacts by three independent first order processes (as shown in figure) with rate constant and for products and respectively. If initially pure was taken in a closed container with atm, then the partial pressure of B (in atm) after from start of experiment :
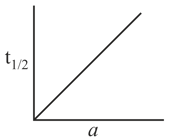
Consider the reaction , graph between half life and initial concentration (a) of the reactant is
Hence, graph between And time will be
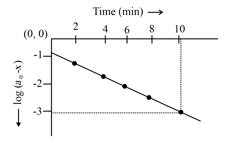
For the first order decomposition of ,
a graph of vs is shown in figure. What is the rate constant ?
Consider the reaction
 .
.
The rate constant for two parallel reactions were found to be and
If the corresponding energies of activation of the parallel reactions are and respectively. What is the apparent (net) overall energy of activation of given reaction.
The following mechanism has been proposed for the exothermic catalyzed complex reaction:
If is much smaller than , the most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
