In the given cell representation which is the negative electrode?
Important Questions on Electrochemistry
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
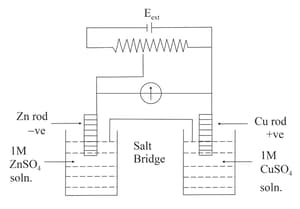
Identify the incorrect statement from the options below for the above cell:
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
Daniell cell converts the chemical energy liberated during the redox reaction to electrical energy.
Identify the anode and cathode in Daniell cell.
At , the of the galvanic cell mentioned below is
,
