MEDIUM
Earn 100
In the given figure, string, spring and pulleys are massless. Block , performing of amplitude and time period . If block remains at rest, and the minimum value of co-efficient of friction between block and surface is . What is the value of ?
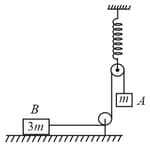

50% studentsanswered this correctly
Important Questions on Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
The position co-ordinates of a particle moving in a coordinate system is given by
and
The speed of the particle is:
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
Which one of the following graph shows correctly the variation with ?
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
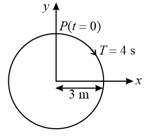
-projection of the radius vector of rotating particle is
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
The displacement time graph of a particle executing SHM is given in figure: (sketch is schematic and not to scale)
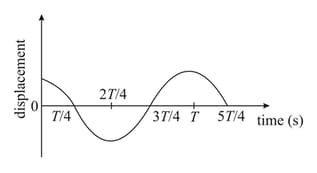
Which of the following statements is/are true for this motion?
(A) The force is zero at
(B) The magnitude of acceleration is maximum at
(C) The speed is maximum at
(D) The is equal to of the oscillation at
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)

