In the human body, vitamin E acts as antioxidant. Identify the functional group or groups that are responsible for anti oxidative properties of this vitamin.

Important Questions on Biochemistry
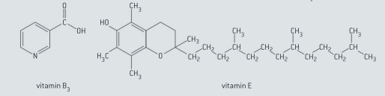
Predict, with reference to functional groups and polarity, whether each of the following vitamins is water-soluble or fat soluble.
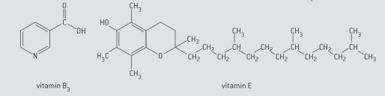
Suggest which vitamin ( or ) must be ingested regularly in small quantities and which one can be taken at much longer intervals but in larger amounts without any detrimental health effects.
The American chemist Linus Pauling, who won two Nobel prizes, promoted the taking of vitamin C as a way of preventing the common cold. One of the functions of vitamin C in the body is as an antioxidant. During the process ascorbic acid, , is converted into dehydroascorbic acid, . Deduce the half-equation to show how vitamin acts as an antioxidant.
The structure of vitamin (ascorbic acid) has some similarities to the structure of carbohydrates. State the name of one functional group that is present both in vitamin and in all carbohydrates.
Predict whether the dietary energy value (in ) of vitamin will be greater than, equal to, or lower than the energy value of glucose.
Vitamins are essential micronutrients that must be obtained from suitable food sources. However, one vitamin can be synthesised in the human body in suffcient quantities even if it is not present in the diet
Discuss whether a non-essential micronutrient can be classified as vitamin.
Explain why vitamin D deficiency in northern countries is more common during the winter.
