EASY
Earn 100
In the long form of periodic table, elements are arranged according to -
(a)Increasing atomic number
(b)Decreasing atomic number
(c)Increasing atomic mass
(d)Decreasing atomic mass
95% studentsanswered this correctly
Important Questions on Periodicity
MEDIUM
EASY
Identify the incorrect match.
| Name IUPAC | Official Name |
| (a) Unnilunium | (i) Mendelevium |
| (b) Unniltrium | (ii) Lawrencium |
| (c) Unnilhexium | (iii) Seaborgium |
| (d) Unununium | (iv) Darmstadtium |
MEDIUM
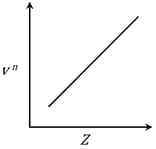
Given Frequency of X-ray emitted
Atomic number
EASY
MEDIUM
EASY
MEDIUM
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
EASY
EASY
Dobereiner's law of triads is not applicable to
EASY
EASY
EASY