EASY
Earn 100
In the plot of molar conductivity vs square root of concentration , following curves are obtained for two electrolytes A and B :
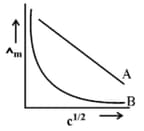
The nature of electrolytes A and B.

(a)A → Strong electrolyte; B → Strong electrolyte
(b)A → Weak electrolyte; B → Strong electrolyte
(c)A → Strong electrolyte; B → Weak electrolyte
(d)A → Weak electrolyte: B → Weak electrolyte
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
HARD
MEDIUM
EASY
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
MEDIUM
EASY
EASY
HARD
(where the constant B is positive)
MEDIUM
EASY
MEDIUM
EASY
HARD
MEDIUM
EASY
HARD
A aqueous solution of has a conductance of when measured in a cell constant . The molar conductivity of this solution is _______ . (Round off to the Nearest Integer)
EASY
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
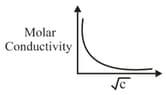
The electrolyte X is :
HARD
MEDIUM

