In the presence of a small amount of phosphorous, aliphatic carboxylic acid reacts with chlorine or bromine to yield a reaction in which, hydrogen is been replaced by halogen. This reaction is known as

Important Questions on Aldehydes, Ketones and Carboxylic Acids
In the following sequence of reactions a compound (molecular formula ) with a straight chain structure gives a carboxylic acid. is :
carboxylic acid
Given below are two statements :
Statement : The nucleophilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
Statement : The nucleophilic addition of hydrogen cyanide to an aldehyde or a ketone yields amine as final product.
In the light of the above statements, choose the most appropriate answer from the options given below :
Two statements are given belwo :
Statement I : The melting point of monocarboxylic acid with even number of carbon atoms is higher than that of with odd number of carbon atoms acid immediately below and above it in the series.
Statement II : The solubility of monocarboxylic acids in water decreases with increase in molar mass.
Choose the most appropriate option :
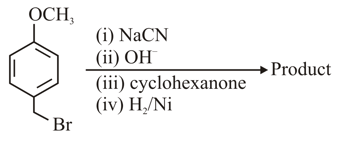
The major product of the above reactions is
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason.
Assertion: A mixture contains benzoic acid and napthalene. The pure benzoic acid can be separated out by the use of benzene.
Reason: Benzoic acid is soluble in hot water.
In the light of the above statements, choose the most appropriate answer from the options given below.
