In the presence of sunlight, benzene reacts with to give product, . The number of hydrogens in is

Important Questions on Hydrocarbons
Given below are two statements. One is labelled as Assertion and the other is labelled as Reason .
Assertion Annulene. Annulene and cis Annulene, are respectively aromatic, not-aromatic and aromatic.
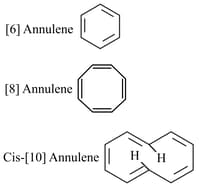
Reason : Planarity is one of the requirements of aromatic systems.
In the light of the above statements, choose the most appropriate answer from the options given below.
Major product '' of the following reaction sequence is
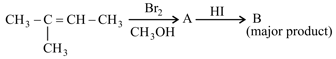
Choose the correct option for the following reactions.
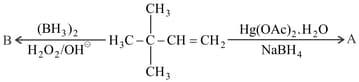
Arrange the following in increasing order of reactivity towards nitration
A. -xylene
B. bromobenzene
C. mesitylene
D. nitrobenzene
E. benzene
Choose the correct answer from the options given below
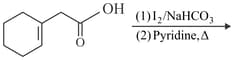
Find out the major product for the above reaction.
In the following given reaction '' is

