MEDIUM
JEE Main
IMPORTANT
Earn 100
In the reaction , which of the graphs is/are correct?
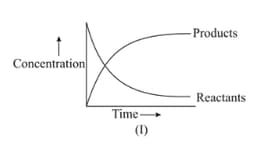
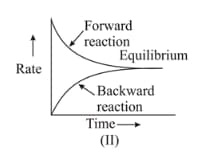
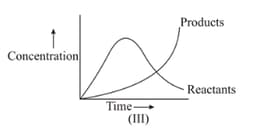



(a)
(b)
(c)
(d)
81.58% studentsanswered this correctly

Important Questions on Chemical Equilibrium
EASY
JEE Main
IMPORTANT
For the reaction , variation of concentration is plotted against time. The time at which the equilibrium establishes is as shown:
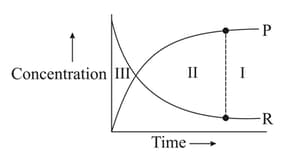
Which of the following regions show(s) equilibrium?
MEDIUM
JEE Main
IMPORTANT
An aquatic species needs at least of for their survival. The solubility of in the water at and pressure is . The partial pressure of above water (in the atmosphere at ) needed for the survival of species is:
EASY
JEE Main
IMPORTANT
In a reversible reaction , the initial concentration of and are and , respectively, in moles per litre and the equilibrium concentration are and , respectively. Express in terms of and .
MEDIUM
JEE Main
IMPORTANT
In the system, is at equilibrium. More water vapour is added to re-establish the equilibrium. The pressure of water vapour is doubled. What is the factor by which the pressure of is changed?
EASY
JEE Main
IMPORTANT
At a particular temperature and atmospheric pressure, the solid and liquid phases of a pure substance can exist in equilibrium. Which of the following term defines the temperature?
HARD
JEE Main
IMPORTANT
Select the correct statements.
EASY
JEE Main
IMPORTANT
Which are heterogeneous system?
MEDIUM
JEE Main
IMPORTANT
Select the correct statements:
