MEDIUM
Earn 100
In the reaction between iodide ion and hydrogen peroxide, what is the role of iodide ion?
(a)
Oxidizing agent
(b)
Reducing agent
(c)
Catalyst
(d)
Solvent
62.12% studentsanswered this correctly
Important Questions on Physical Chemistry Experiments
EASY
The number of octahedral voids per lattice site in a lattice is_. (Rounded off to the nearest integer)
MEDIUM
A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is doubled?
HARD
On which of the following parameters does the rate constant of a reaction depend?
(i) time, (ii) concentration of reactants, (iii) temperature, and (iv) catalyst
MEDIUM
The rate of a first-order reaction is at second and at seconds after initiation of the reaction. The half-life period of the reaction is:
MEDIUM
The rate of a gaseous reaction is given by the expression If the volume of vessel is reduced to one half of the initial volume, the reaction rate as compared to original rate is
EASY
The addition of a catalyst during a chemical reaction alters which of the following quantities?
MEDIUM
Name the factors that affect the rate of a reaction. Explain in detail.
HARD
For an elementary chemical reaction, , the expression for is:
EASY
How will the rate of reaction be affected when surface area of the reactant is reduced?
EASY
In mechanism of any reaction, the step going in the slowest manner is called _____
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
of is mixed with of to form an ester. The rate of the reaction with respect to the initial rate when each solution is diluted with an equal volume of water will be
EASY
Decomposition of exhibits a rate constant of . How many years are required for the decomposition of of into ?
HARD
According to the Arrhenius equation,
MEDIUM
For the reaction, products, when the concentration of and both were doubled, the rate of the reaction increased from to When the concentration of alone is doubled, the rate increased from to
Which one of the following statements is correct?
HARD
For a first order reaction at constant volume and 300 K, the total pressure at the beginning and at time are , respectively. Initially, only A is present with concentration , and is the time required for the partial pressure of A to reach of its initial value. The correct option(s) is (are)
(Assume that all these gases behave as ideal gases)
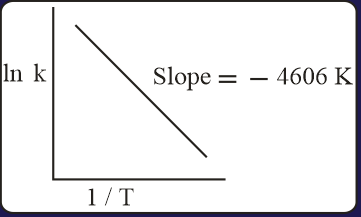
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
EASY
Zinc piece and zinc powder are taken in two test tubes and equal amount of dil. is added.
(a) In which test tube does the reaction proceed faster?
(b) Give reason.
(c) Give an instance in daily life, where such condition is made use.
EASY
Which one of the factors does not affect rate of the reaction?
EASY
The decomposition of phosphine on tungsten at low pressure is a first-order reaction. It is because the

