EASY
JEE Main
IMPORTANT
Earn 100
In the sulphur estimation, of an organic compound gave of barium sulfate. The percentage of sulphur in the compound is. (Nearest integer) (Atomic Mass of )
100% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
JEE Main
IMPORTANT
Which among the following is the strongest acid?
MEDIUM
JEE Main
IMPORTANT
of an organic compound was analysed by Kjeldahl's method for the estimation of nitrogen. If the percentage of nitrogen in the compound was found to be , then....... of would have been neutralized by the ammonia evolved during the analysis.
MEDIUM
JEE Main
IMPORTANT
Which one among the following resonating structures is not correct?
MEDIUM
JEE Main
IMPORTANT
Which of the following compound is added to the sodium extract before addition of silver nitrate for testing of halogens?
MEDIUM
JEE Main
IMPORTANT
The correct order of acid character of the following compounds is :
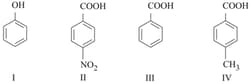
HARD
JEE Main
IMPORTANT
Complete combustion of of an oxygen containing compound gave of and of The percentage of oxygen in the organic compound is :
MEDIUM
JEE Main
IMPORTANT
Using the provided information in the following paper chromatogram:
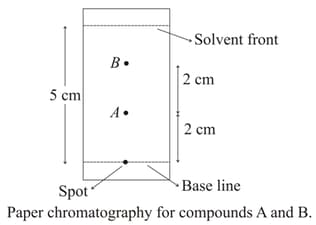
The calculated value of .
MEDIUM
JEE Main
IMPORTANT
The formula of a gaseous hydrocarbon which requires 6 times of its own volume of for complete oxidation and produces times its own volume of is The value of is ________.
