EASY
JEE Main
IMPORTANT
Earn 100
In thermodynamics, heat and work are :
(a)Path functions
(b)Intensive thermodynamic state variables
(c)Extensive thermodynamic state variables
(d)Point functions
69.57% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
For an ideal heat engine, the temperature of the source is . In order to have efficiency the temperature of the sink should be __________. (Round off to the nearest integer)
EASY
JEE Main
IMPORTANT
A Carnot's engine working between and has a work output of per cycle. The amount of heat energy supplied to the engine from the source in each cycle is:
EASY
JEE Main
IMPORTANT
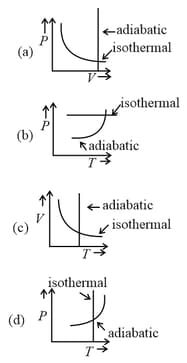
Which one is the correct option for the two different thermodynamic processes ?
MEDIUM
JEE Main
IMPORTANT
The diagram of a diatomic ideal gas system going under cyclic process as shown in figure. The work done during an adiabatic process is (use :
EASY
JEE Main
IMPORTANT
For an adiabatic expansion of an ideal gas, the fractional change in its pressure is equal to (where is the ratio of specific heats):
MEDIUM
JEE Main
IMPORTANT
An ideal gas in a cylinder is separated by a piston in such a way that the entropy of one part is and that of the other part is . Given that . If the piston is removed then the total entropy of the system will be:
EASY
JEE Main
IMPORTANT
Match List with List
| List | List | ||
| (a) | Isothermal | (i) | Pressure constant |
| (b) | Isochoric | (ii) | Temperature constant |
| (c) | Adiabatic | (iii) | Volume constant |
| (d) | Isobaric | (iv) | Heat content is constant |
Choose the correct answer from the options given below:
EASY
JEE Main
IMPORTANT
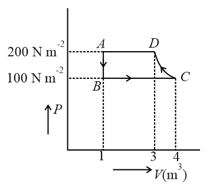
mole of a perfect gas undergoes a cyclic process (see figure) consisting of the following processes.
Isothermal expansion at temperature so that the volume is doubled from to and pressure changes from to
Isobaric compression at pressure to initial volume
Isochoric change leading to change of pressure from to
Total work done in the complete cycle is: