EASY
NEET
IMPORTANT
Earn 100
In which of the figure no heat exchange between the gas and the surroundings will take place, if the gas is taken along a curve?
(curves are isothermal and adiabatic)
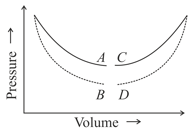

(a)
(b)
(c)
(d)
28.57% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
In the following figures, four curves are shown the curves are:-
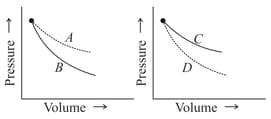
MEDIUM
NEET
IMPORTANT
A vessel contains an ideal monatomic gas that expands at constant pressure when heat is given to it. Then the work done in expansion is:
MEDIUM
NEET
IMPORTANT
One mole of an ideal gas at temperature expands according to the law (constant). The work done by the gas till the temperature of gas becomes is
MEDIUM
NEET
IMPORTANT
When an ideal diatomic gas is heated at constant pressure, the fraction of the heat energy supplied which increases the internal energy of the gas is.
EASY
NEET
IMPORTANT
One mole of an ideal monoatomic gas is heated at a consant pressure of one atmosphere from 0ºC to 100ºC. Then the change in the internal energy is
EASY
NEET
IMPORTANT
The specific heat of a gas at constant pressure is more than that of the same gasat constant volume because :-
MEDIUM
NEET
IMPORTANT
Air is filled in a tube of the wheel of a car at 27ºC and 2 atm pressure if the tube is suddenly bursts, the final temperature of air will be :-
EASY
NEET
IMPORTANT
Specific heat of a gas undergoing adiabatic change is
