EASY
Earn 100
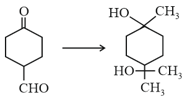
In which of the following decreases if there can be some intramolecular rearrangement?
(a)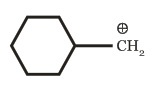

(b)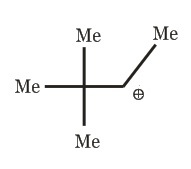

(c)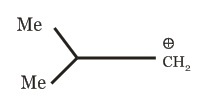

(d)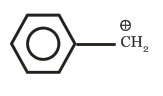

50% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
Which of the following carbocations is most stable?
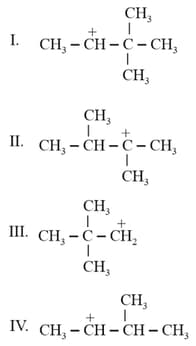
MEDIUM
The compound that is most difficult to protonate is:
EASY
The lower stability of ethyl anion compared to methyl anion and the higher stability of ethyl radical compared to methyl radical, respectively, are due to
EASY
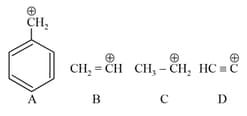
The correct order of stability of given carbocation is:
EASY
The order of stability of the following carbocations:
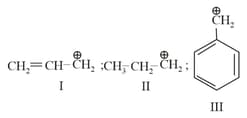
MEDIUM
Choose the correct statement regarding the formation of carbocations A and B given :-
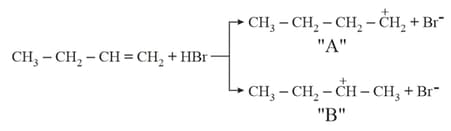
EASY
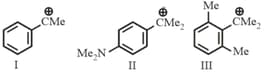
For the following carbocations, the correct order of stability is
I.
II.
III.
MEDIUM
Assertion (A): Tertiary carbocations are more reactive than secondary and primary carbocations.
Reason (R): Hyper conjugation, as well as inductive effect due to additional alkyl groups stabilise tertiary carbocations.
HARD
A solution of in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of :
MEDIUM
In which of the following compounds, the bond ionization shall give most stable carbonium ion?
EASY
The most stable carbocation among the following is
HARD
The major products and in the following reactions are
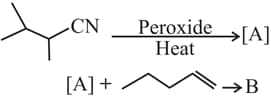
EASY
The decreasing order of the stability of the following carbocations is
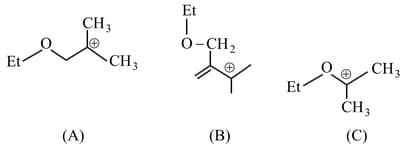
MEDIUM
The stability order of the above carbocations is
MEDIUM
The decreasing order of stability of the given carbocations is

MEDIUM
Carbocations are important intermediates in organic reactions, they are often stabilised by resonance, inductive effect and hyperconjugation. The cation that can be stabilised by hyperconjugation is
MEDIUM
Consider the following carbocations
(a)
(b)
(c)
(d)
The stability sequence follows the order
MEDIUM
The stability of carbocations

follows the order
MEDIUM
Define ambident nucleophile with an example.
MEDIUM
The correct sequence of reagents for the following conversion will be