MEDIUM
NEET
IMPORTANT
Earn 100
In which of the following molecules, the central atom expands its covalency by excitation but not its octet?
(a)
(b)
(c)
(d)
(a)b and d
(b)a and c
(c)b and c
(d)b, c and d
34.74% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Maximum number of lone pairs is present in Lewis dot structure of which compound?
EASY
NEET
IMPORTANT
The ratio of to bonds in naphthalene is
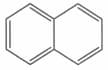
MEDIUM
NEET
IMPORTANT
What is true about molecule?
It has two lone pairs.
It has one dative bond.
It has two bonds.
It has one bond.
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
