HARD
JEE Main
IMPORTANT
Earn 100
In which of the following pairs, electron gain enthalpies of constituent elements are nearly the same or identical?
(A) and
(B) and
(C) and
(D) and
Choose the correct answer from the options given below:
(a) only
(b) only
(c) only
(d) only
36.94% studentsanswered this correctly

Important Questions on Classification of Elements and Periodicity in Properties
EASY
JEE Main
IMPORTANT
The correct decreasing order for metallic character is
HARD
JEE Main
IMPORTANT
The first ionization enthalpy of and , respectively, are: and . The first ionization enthalpy of is
MEDIUM
JEE Main
IMPORTANT
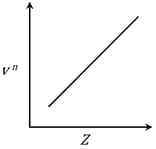
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
EASY
JEE Main
IMPORTANT
Which of the following represents the correct order of metallic character of the given elements?
EASY
JEE Main
IMPORTANT
Total number of acidic oxides among and is _____ .
EASY
JEE Main
IMPORTANT
Match List - I with List - II
| LIST-I (Atomic number) |
LIST-II (Block of periodic table) |
||
| (A) | I. | -block | |
| (B) | II. | -block | |
| (C) | III. | -block | |
| (D) | IV. | -block | |
Choose the correct answer from the options given below:
MEDIUM
JEE Main
IMPORTANT
The correct increasing order of the ionic radii is
MEDIUM
JEE Main
IMPORTANT
For electron gain enthalpies of the elements denoted as , the incorrect option is :