In which part , air and gas(petrol) mixes in internal combustion engine?
Important Questions on Sets, Relations and Functions
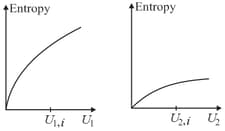
Graphs below show the entropy versus energy of two systems and at constant volume. The initial energies of the systems are indicated by and respectively. Graphs are drawn to the same scale. The systems are then brought into thermal contact with each other. Assume that, at all times the combined energy of the two systems remains constant. Choose the most appropriate option indicating the energies of the two systems and the total entropy after they achieve the equilibrium.
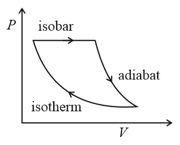
The efficiency of the cycle shown below in the figure (consisting of one isobar, one adiabat and one isotherm) is The ratio, between the highest and lowest temperatures attained in this cycle obeys (the working substance is a ideal gas):-
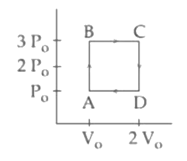
An engine operates by taking a monatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to
Step It is first compressed adiabatically from volume to
Step then expanded isothermally to volume
Step then expanded adiabatically to volume
Step then compressed isothermally to volume If the efficiency of the above cycle is then is

