MEDIUM
NEET
IMPORTANT
Earn 100
In which process, net work done by an ideal gas is zero:
(a)Cyclic
(b)Isobaric
(c)Free expansion
(d)Adiabatic
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
plots for two gases during an adiabatic process are given in the figure:
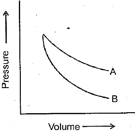
Plot and plot should correspond to: (Assume ideal behaviour).
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
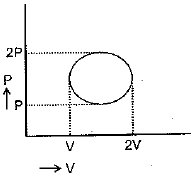
For this cyclic process change in enthalpy of ideal gas is:
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
