MEDIUM
Earn 100
In which year did the Deccan Riots take place?
(a)1857
(b)1875
(c)1885
(d)1867
50% studentsanswered this correctly
Important Questions on Amines
HARD
EASY
HARD
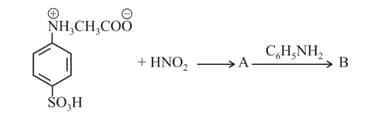
MEDIUM
MEDIUM
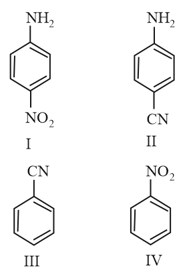
Which of the following relations is correct?
MEDIUM
HARD
The major product of the following reaction is
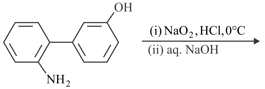
EASY
HARD
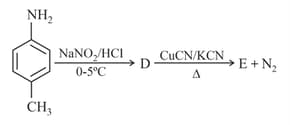
In a set of reactions nitrotoluene yielded a product
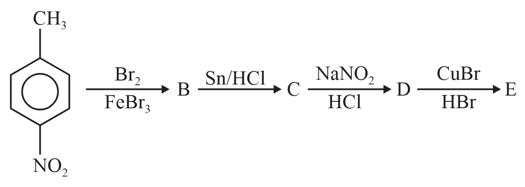
Product would be:
EASY
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
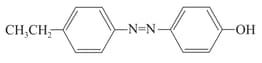
EASY
MEDIUM