EASY
NEET
IMPORTANT
Earn 100
Increase in temperature of a gas filled in a container will lead to
(a)increase in its mass
(b)increase in its kinetic energy
(c)decrease in its pressure
(d)decrease in intermolecular distance
73.08% studentsanswered this correctly

Questions featured in Previous Year Papers on Heat & Thermodynamics
MEDIUM
NEET
IMPORTANT
Match Column - I and Column - II and choose the correct match from the given choices.
| Column - I | Column - II |
| (A) Root mean square speed of gas molecules | |
| (B) Pressure exerted by ideal gas | |
| (C) Average kinetic energy of a molecule | |
| (D) Total internal energy of of a diatomic gas |
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
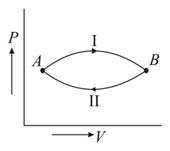
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
HARD
NEET
IMPORTANT
HARD
NEET
IMPORTANT
