EASY
JEE Main/Advance
IMPORTANT
Earn 100
Indicate what is wrong with each of the following Lewis structures. Replace each with a more acceptable structure.
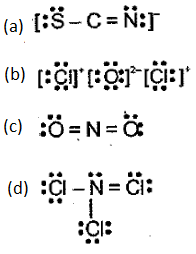
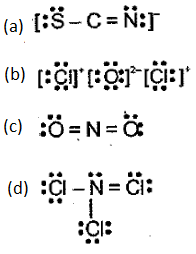

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main/Advance
IMPORTANT
In how many of the following species, the central atoms have two lone pairs of electrons?
(i)
(ii)
(iii)
(iv)
(V)
(vi)
(vii)
(viii)
(ix)
EASY
JEE Main/Advance
IMPORTANT
How many compounds violate octet rule?
(i)
(ii)
(iii)
(iv)
(v)
(vi)
(vi) friii)
EASY
JEE Main/Advance
IMPORTANT
Write the reason for the violation of octet rule by various molecules?
MEDIUM
JEE Main/Advance
IMPORTANT
How many types of bond lengths are present in
MEDIUM
JEE Main/Advance
IMPORTANT
Compare bond length of bond in and .
MEDIUM
JEE Main/Advance
IMPORTANT
Find number of sigma bonds and pi bonds in
HARD
JEE Main/Advance
IMPORTANT
Draw the type of overlaps between
(a)
(b)
(c) and
(d) and
(e) and
(f) and
(g) s and
(h) and
(i) and
(j) and
(k) and
(1) and
(m) and
(n) and
(o) and not
if internuclear axis is z-axis. Identify them as bond wherever bond is formed.
EASY
JEE Main/Advance
IMPORTANT
Among the following which property is commonly exhibited by a covalent molecule.
