Industrial reaction conditions for the production of ammonia is as shown below in the graph:
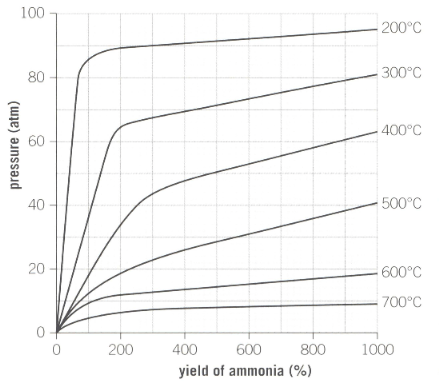
Explain with reference to the position of the equilibrium why increasing the pressure of this closed system favours the forward reaction.


Important Questions on Balance
The Haber's process describes the industrial production of ammonia gas on a large scale: . Predict the effect of the following change on the position of the equilibrium in the process.
Nitrogen gas is added to the system at equilibrium.
The Haber's process describes the industrial production of ammonia gas on a large scale: . Predict the effect of the following change on the position of the equilibrium in the process.
Hydrogen gas is removed from the system at equilibrium.
The Haber's process describes the industrial production of ammonia gas on a large scale: . Predict the effect of the following change on the position of the equilibrium in the process.
The pressure of the system is decreased.
The Haber's process describes the industrial production of ammonia gas on a large scale: . Predict the effect of the following change on the position of the equilibrium in the process.
The temperature of the system is increased.
