Industrial reaction conditions for the production of ammonia is as shown below in the graph:
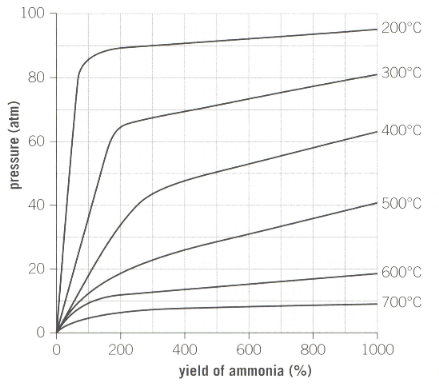
What temperature achieves the highest yield of ammonia?


Important Questions on Balance
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
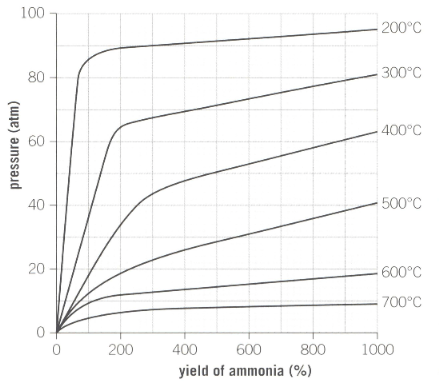
Describe how the yield changes as the pressure is increased.
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
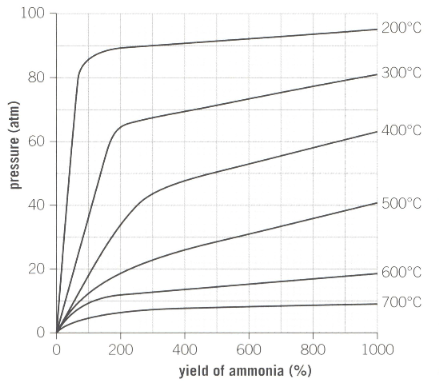
The ideal temperature used by Haber is approximately . Outline the reasons why a higher temperature would be used to increase production of ammonia instead of a lower temperature.
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
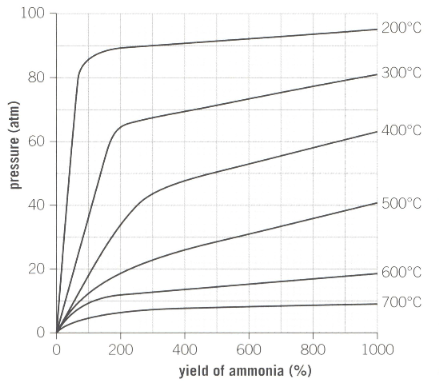
A pressure of is used during the process. Why does industry not use a much higher pressure to maximise yield?
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
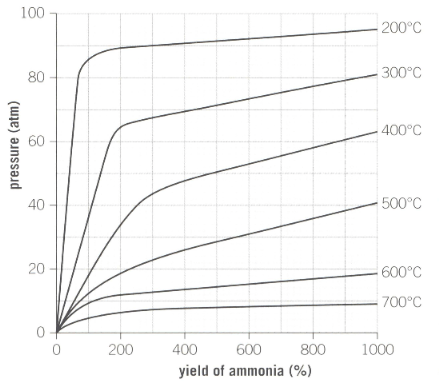
Explain with reference to the position of the equilibrium why increasing the pressure of this closed system favours the forward reaction.
