Instant cold packs can be made using ammonium nitrate and water. The pack contains a small bag of solid ammonium nitrate. This bag is inside another bag, which is full of water. When you squeeze the outer bag the smaller bag bursts. The ammonium nitrate dissolves in water and the pack becomes cold.
Explain how you know this.

Important Questions on Energy Transfers in Chemistry
A student planned an experiment to find out which substance would produce the biggest temperature decrease when added to either water or an acid. The chemicals she had access to were:
- ammonium nitrate solid
- sodium hydroxide solution
- citric acid solution
- sodium hydrogen carbonate solution
- water
- dilute hydrochloric acid
State one problem the student would have in making this fair test.
A student planned an experiment to find out which substance would produce the biggest temperature decrease when added to either water or an acid. The chemicals she had access to were:
- ammonium nitrate solid
- sodium hydroxide solution
- citric acid solution
- sodium hydrogen carbonate solution
- water
- dilute hydrochloric acid
How would you improve this method.
Jonathan carried out an experiment to measure the temperature change when two solutions are mixed. His results are shown below.
| Reaction | Temperature at start | Temperature at end |
| Sodium hydrogen carbonate solution + Citric acid | ||
| Sodium hydroxide solution + Citric acid | ||
| Dilute hydrochloric acid + Citric acid solution |
What were the dependent and independent variables in this experiment?
Jonathan carried out an experiment to measure the temperature change when two solutions are mixed. His results are shown below.
| Reaction | Temperature, at start | Temperature, at end |
| Sodium hydrogen carbonate solution + Citric acid | ||
| Sodium hydroxide solution + Citric acid | ||
| Dilute hydrochloric acid + Citric acid solution |
State one variable that Jonathan should have controlled in this experiment.
Jonathan carried out an experiment to measure the temperature change when two solutions are mixed. His results are shown below.
| Reaction | Temperature , at start | Temperature , at end |
| Sodium hydrogen carbonate solution + Citric acid | ||
| Sodium hydroxide solution + Citric acid | ||
| Dilute hydrochloric acid + Citric acid solution |
Which one of these reactions would be the most suitable for use in making an instant cold pack? Explain your answer.
Jonathan carried out an experiment to measure the temperature change when two solutions are mixed. His results are shown below.
| Reaction | Temperature , at start | Temperature , at end |
| Sodium hydrogen carbonate solution + Citric acid | ||
| Sodium hydroxide solution + Citric acid | ||
| Dilute hydrochloric acid + Citric acid solution |
What type of the reaction is second reaction?
The diagram shows the particles in a liquid that is evaporating.
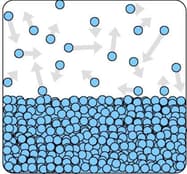
How can you tell from the diagram that the liquid is evaporating?
The diagram shows the particles in a liquid that is evaporating.
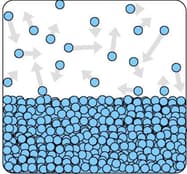
Explain why evaporation causes the temperature of the liquid to decrease?
