MEDIUM
11th CBSE
IMPORTANT
Earn 100
Ionic radii vary in
(a)inverse proportion to the effective nuclear charge.
(b)inverse proportion to the square of effective nuclear charge.
(c)direct proportion to the screening effect.
(d)direct proportion to the square of screening effect.
50% studentsanswered this correctly

Important Questions on Classification of Elements and Periodicity in Properties
MEDIUM
11th CBSE
IMPORTANT
An element belongs to period and group- of the periodic table. Which of the following properties will be shown by the element?
HARD
11th CBSE
IMPORTANT
Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
MEDIUM
11th CBSE
IMPORTANT
All transition elements are -block elements, but all -block elements are not transition elements. Explain.
MEDIUM
11th CBSE
IMPORTANT
Identify the group and valency of the element having the atomic number . Also, predict the outermost electronic configuration and write the general formula of its oxide.
MEDIUM
11th CBSE
IMPORTANT
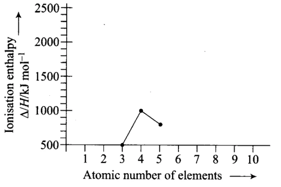
Ionisation enthalpies of elements of the second period are given below
Ionisation enthalpy
Match the correct enthalpy with the elements and complete the graph given in the figure. Also, write symbols of elements with their atomic number.
MEDIUM
11th CBSE
IMPORTANT
Among the elements and , which element has the highest first ionisation enthalpy?
MEDIUM
11th CBSE
IMPORTANT
Among the elements and , which element has the most metallic character? Justify your answer in each case.
MEDIUM
11th CBSE
IMPORTANT
Write four characteristic properties of -block elements.
