HARD
Earn 100
Iron crystalizes in with an edge length of . If it contains Schottky defects, calculate its approximate density [ of ]
(a)
(b)
(c)
(d)
22.73% studentsanswered this correctly
Important Questions on Solid State
MEDIUM
(Given atomic weight of
HARD
MEDIUM
MEDIUM
HARD
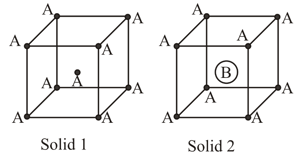
Consider the bcc unit cells of the solids and with the position of atoms as shown below. The radius of atom is twice that of atom The unit cell edge length is more in solid than in . What is the approximate packing efficiency in solid ?
EASY
MEDIUM
(Avogadro constant )
HARD
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
EASY
The vacant space in bcc lattice unit cell is:
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
The packing efficiency of the face centered cubic (FCC), body centered cubic (BCC) and simple/primitive cubic (PC) lattices follows the order
HARD

