MEDIUM
7th CBSE
IMPORTANT
Earn 100
Is it possible to raise the temperature of water by heating it in a container made-up of paper? [Temperature required to burn the paper is more than ]

Important Questions on Heat
MEDIUM
7th CBSE
IMPORTANT
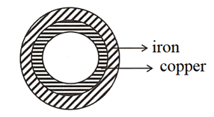
Rings made-up of copper and iron are fixed as shown in the figure. On heating the rings, explain what happens to them, if the thickness of the rings are negligible.
MEDIUM
7th CBSE
IMPORTANT
MEDIUM
7th CBSE
IMPORTANT
Explain why a clinical thermometer is exclusively used to measure the human body temperature and a laboratory thermometer is used to measure the temperatures of different substances but not to measure the human body temperature.
HARD
7th CBSE
IMPORTANT
HARD
7th CBSE
IMPORTANT
HARD
7th CBSE
IMPORTANT
MEDIUM
7th CBSE
IMPORTANT
MEDIUM
7th CBSE
IMPORTANT
