Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
chlorine, Cl2

Important Questions on Chemical Bonding
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
hydrogen fluoride, HF
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
The V-shaped molecule, sulfur dichloride, SCI2
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
The tetrahedral molecule, chloromethane, CH3Cl
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
The tetrahedral molecule, tetrabromomethane, CBr4
The boiling points of the halogens are:
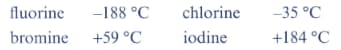
Describe the trend in these boiling points going down Group 17.
The boiling points of the halogens are:
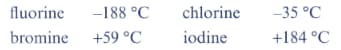
Explain the trend in these boiling points.
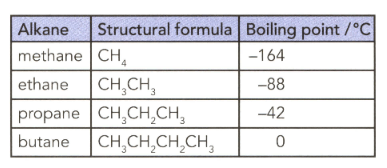
The table lists the formulae and boiling points of some alkanes. Explain the trend in terms of instantaneous dipole-induced dipole forces.