Is there any change in the amount of solute dissolved when the temperature changes?

Important Questions on Solutions
While preparing saturated solutions of various solutes in a definite amount of a given solvent under the same conditions, will the amount of solutes getting dissolved be the same? Try to find out.
Given is a graph that connects the solubility and the temperature of certain salts.
Examine the graph and find out the following:
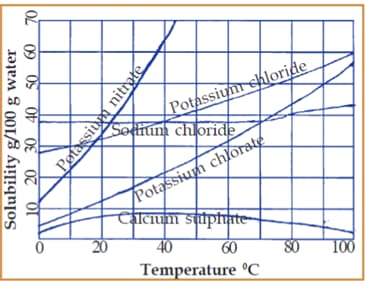
Which substance shows the maximum increase in solubility as temperature increases?
Given is a graph that connects the solubility and the temperature of certain salts.
Examine the graph and find out the following:
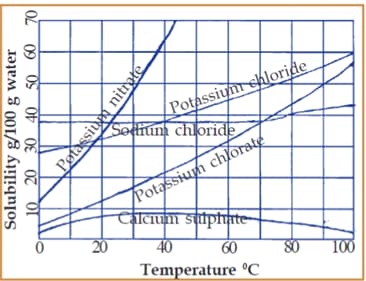
Which salts have the same solubility at a temperature of ?
Given is a graph that connects the solubility and the temperature of certain salts. Examine the graph and find out the following:
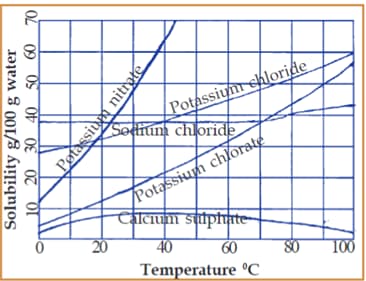
Which substance shows a decrease in solubility with an increase in temperature?
Given is a graph that connects the solubility and the temperature of certain salts. Examine the graph and find out the following:
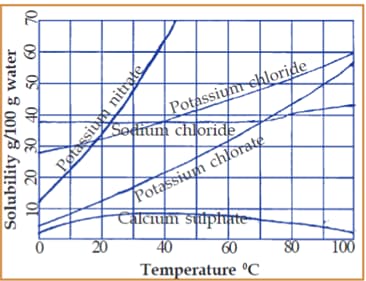
Prepare a note on the influence of temperature on the solubility of substances. Give examples.
All solutions are mixtures. Still, do all mixtures exhibit the same nature?
Let's do an experiment. Take equal amounts of water in three separate beakers. Add copper sulphate crystals in the first, milk in the second and chalk powder in the third beaker. Stir them well. Keep the beakers undisturbed for some time.
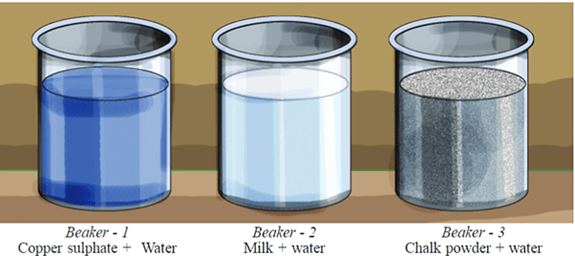
In which of these beakers did the substance settle down?
Let's do an experiment. Take equal amounts of water in three separate beakers. Add copper sulphate crystals in the first, milk in the second and chalk powder in the third beaker.
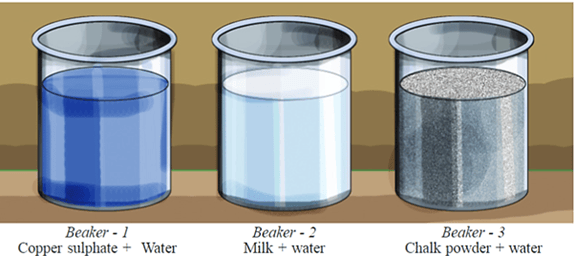
What will you observe if an intense beam of light is passed through the sides of the three beakers? Write your answers in Yes/No in the following table.
| Observation | Beaker- | Beaker- | Beaker- |
| The path of the beam can be observed | |||
| Particles can be observed |
