HARD
Earn 100
Is water an acid?
Important Questions on Acids and Bases
MEDIUM
The bulb is not glowing in this experimental setup. Because:
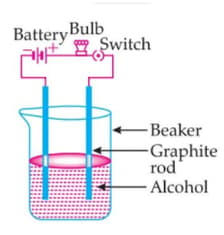
MEDIUM
EASY
HARD
Differentiate between the following pair based on the information given in the bracket.
- Acid and Alkali (formation of the type of ions)
EASY
EASY
Which of the following is a strong acid?
MEDIUM
Four solutions A, B, C, and D have pH 2, 6. 7, and 13 respectively:
Which solution will have the highest number of hydronium ions?
MEDIUM
EASY
Give the appropriate term defined by the statement given below:
The substance that releases hydronium ion as the only positive ion when dissolved in water
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
HARD

