EASY
11th CBSE
IMPORTANT
Earn 100
Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs.
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
11th CBSE
IMPORTANT
Polarity in a molecule and the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
HARD
11th CBSE
IMPORTANT
The types of hybrid orbitals of nitrogen in , and respectively are expected to be
MEDIUM
11th CBSE
IMPORTANT
Hydrogen bonds are formed in many compounds e.g., , , . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is:
EASY
11th CBSE
IMPORTANT
In ion the formal charge on the oxygen atom of bond is
MEDIUM
11th CBSE
IMPORTANT
In ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
MEDIUM
11th CBSE
IMPORTANT
Which of the following species has tetrahedral geometry?
EASY
11th CBSE
IMPORTANT
Number of bonds and bonds in the following structure is–
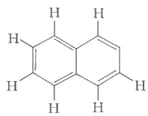
HARD
11th CBSE
IMPORTANT
Which molecule/ion out of the following does not contain unpaired electrons?
