HARD
Earn 100
KBr crystallises in NaCl type of crystal lattice and its density is 2.75 . Number of unit cells of KBr present in a 1.00 grain of KBr are
(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Solid State
MEDIUM
EASY
EASY
EASY
HARD
EASY

EASY
EASY
EASY
MEDIUM
HARD
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
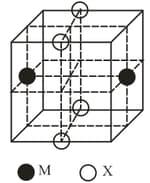
MEDIUM
MEDIUM
MEDIUM
(a) Aluminium crystallizes in a cubic close-packed structure. Its metallic radius is . What is the length of the side of the unit cell?
(b) Why is potassium chloride sometimes violet instead of pure white?
MEDIUM
EASY
EASY
EASY

