EASY
JEE Main/Advance
IMPORTANT
Earn 100
can be used in salt bridge as the electrolyte in which of the following cells?
(a)
(b)
(c)
(d)
25% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main/Advance
IMPORTANT
Consider the following values
Under standard conditions, the potential for the reaction is
MEDIUM
JEE Main/Advance
IMPORTANT
Given standard electrode potentials:
The standard electrode potential for .
MEDIUM
JEE Main/Advance
IMPORTANT
Then for , will be:
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
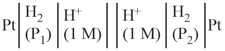 (where and are pressures) the cell reaction will be spontaneous, if :
(where and are pressures) the cell reaction will be spontaneous, if :EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
