EASY
Earn 100
L shell corresponds to the _____ orbit in an atom.
(a)second
(b)first
(c)fourth
(d)third
50% studentsanswered this correctly
Important Questions on What is inside the atom?
MEDIUM
MEDIUM
MEDIUM
MEDIUM
An isoelectronic species are
EASY
EASY
MEDIUM
MEDIUM
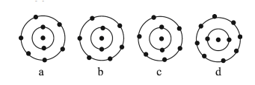
The schematic atomic structure of four elements is given below. Observe and choose the right statement.
EASY
MEDIUM
MEDIUM
MEDIUM
Identify the sets of quantum numbers which are not possible?
EASY
MEDIUM
Match the following :
List -I :
(a) Frequency of distribution of the emitted radiation from a black body
(b) Spin quantum numbers(ms)
(c) Angular Momentum
(d) All orbital have equal energy
List - II :
(i) degeneracy
(ii) temperature dependent
(iii) vector quantity
(iv) mass times velocity times radius
Codes:
EASY
MEDIUM
EASY
EASY
EASY
EASY

