Lattice energy of an ionic compound depends upon :

Important Questions on Chemical Bonding and Molecular Structure
Consider the following statements
is pyramidal about each atom.
is pyramidal about the atom and bent about the atom.
is trigonal about the carbon atom (attached to and ) and out of these select the correct one.
Which of the following statements are correct?
()
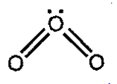 structure is not allowed because octet around 'O' can not be expanded.
structure is not allowed because octet around 'O' can not be expanded.
() is ionic compound.
() In motecule, the highest occupied molecular orbital is molecular orbital.
() The repulsion is stronger than repulsion.
simple ionic compounds show isomerism and isomorphism due to the directional nature of the electrovalent bond.
Covalent bond formed by hybrid orbitals are more stronger than those of formed by pure atomic orbitals.
anion has
bonds of unequal length
shybridisation of carbon atom with same bond angles
The number of lone pair of electrons present on in and are and , respectively.
Which of the following is not an ionic compound?
