Least stable carbocation among the following is
Important Points to Remember in Chapter -1 - Organic Chemistry : Some Basic Principles and Techniques from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
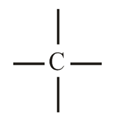
1. Tetravalency of Carbon:
(i) The atomic number of Carbon is and it has valence electrons. Thus, carbon is always tetra covalent, i.e., it forms covalent bonds with other atoms.
(ii) Catenation: The self-linking property of carbon is known as catenation. This is the main reason for the existence of so many compounds.
(iii) Functional Group: An atom or a group of atoms joined in a specific manner, which provides certain characteristic chemical properties to the organic compounds, is called as a functional group.
(iv) Homologues: A group or a series of an organic compound each containing a characteristic functional group from a homologous series. The members of the series are called "homologous".
2. IUPAC Nomenclature of Organic Compounds:
Following rules are used to write the IUPAC name of an organic compound.
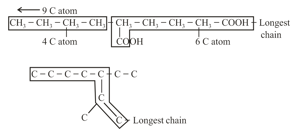
(i) Longest chain rule: The chain containing the principal functional group, secondary functional group and multiple bonds, as many as possible is the longest possible chain.
(ii) In the absence of a functional group, the secondary group and the multiple bonds, the chain containing the maximum number of -atoms will be the longest possible chain, for example:
(iii) Choose the word root from the table given below for the longest possible chain.
| Chain length | Word root | Chain length | Word root |
|
Meth- |
Hept |
||
|
Eth- |
Oct |
||
|
Prop- |
Non |
||
|
But- |
Dec |
||
|
Pent- |
Undec |
||
|
Hex- |
Dodec |
(iv) Lowest number rule: Numbering is done in such a way that:
(a) Branching, if present, gets the lowest number.
(b) The sum of numbers of side chains is lowest.
(c) The principal functional group gets the lowest number.
(v) Select the principal functional group from the preference series:
Functional groups other than the principal functional group are called as substituents.
(vi) Naming the prefixes and suffixes: Prefix represents the substituent and suffix is used for the principal functional group.
(a) Primary prefixes are cyclo, bicyclo, di, tri, tetra, tetrakis etc.
(b) Secondary prefixes are tabulated below:
| Substituent | Prefix | Substituent | Prefix |
| Fluoro | diazo | ||
| Chloro | nitroso | ||
| Bromo | nitro |
(c) Primary suffixes are ene, ane or yne used for double, single and triple bonds respectively.
(d) Secondary suffixes axe tabulated below:
| Functional group | Structure | Prefix | Suffix |
| Carboxylic acid | 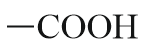 |
Carboxy | -oic acid |
| Sulphonic acid | 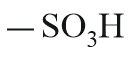 |
Sulpho | sulphonic acid |
| Ester | 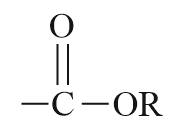 |
Alkoxy carbonyl | alkyl…oate |
| Acid chloride | 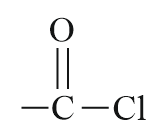 |
Chloroformyl or Chlorocarbonyl | -oyl chloride |
| Acid amide | 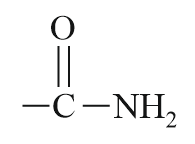 |
Carbamoyl/ Amido | -amide |
| Carbonitrile/Cyanide |  |
Cyano | nitrile |
| Aldehyde | 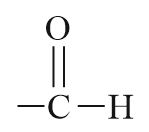 |
Formyl or oxo | -al |
| Ketone | 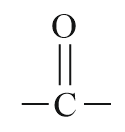 |
Keto or oxo | -one |
| Alcohol |  |
Hydroxy | -ol |
| Thio alcohol | 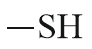 |
Mercapto | thiol |
| Amine |  |
Amino | amine |
| Ether | 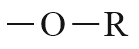 |
Alkoxy | - |
| Oxirane | 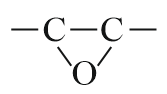 |
Epoxy | - |
| Nitro derivative |  |
Nitro | - |
| Nitroso derivative |  |
Nitroso | - |
| Double bond | 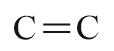 |
- | ene |
| Triple bond | 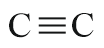 |
- | yne |
(vii) Primary prefix + secondary prefix Word root primary suffix secondary suffix
For example:
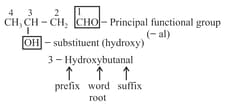
(viii) If more than two similar functional groups are present, all the group carbon are excluded from principal chain. For example:
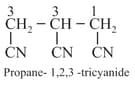
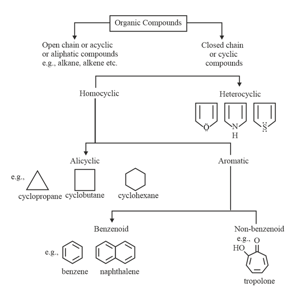
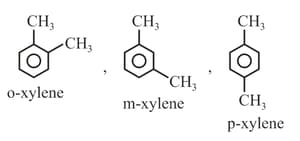
(ix) Naming of Aromatic compounds: accepted their common trivial names, for example

3. Isomerism:
(i) Structural isomerism is a form of isomerism in which molecules with the same molecular formula have atoms bonded together in different orders.
(ii) Types of structural isomerism:
(a) Chain isomerism: This type of isomerism is due to the difference in the arrangement of the carbon atoms constituting the chain.
Key points: Parent carbon chain or side chain should be different.
For example: : and isobutane.
(b) Positional isomerism: It occurs when functional groups or multiple bonds or substituents are in different positions on the same carbon chain.
Key point: Parent carbon chain remains the same and the substituent, multiple bonds and the functional group changes its position.
For example:
(c) Functional isomerism: It occurs when compounds have the same molecular formula but different functional groups.
For example:

(d) Metamerism: This type of isomerism occurs when the isomers differ with respect to the nature of the alkyl groups around the same polyvalent functional group.
For example:
(e) Ring-chain isomerism: In this type of isomerism, one isomer is open chain, but another is cyclic.
For example:

4. Tautomerism:
(i) This type of isomerism is due to spontaneous interconversion of two isomeric forms into each other with different functional groups in dynamic equilibrium.
(ii) Conditions:
(a) Presence of a
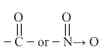
(b) Presence of at least one atom which is attached to a saturated -atom.
For example: Acetoacetic ester.

(iii) Enol content
(a) Acidity of of keto form.
(b) Intramolecular H-Bonding in enol form.
(c) Resonance in enol form.
(d) Aromatisation in enol form.
5. Stereoisomerism:
Compounds with the same molecular formula and structural formula but having differences in the spatial arrangement of atoms or groups are called as stereoisomers and the phenomenon is known as stereoisomerism.
6. Geometrical isomerism:
It is due to restricted rotation and is observed in following systems:
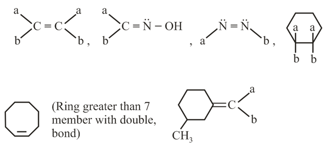
(i) Cis-trans isomerism: The cis compound is the one with the same groups on the same side of the bond, and the trans has the same groups on the opposite sides. Both isomers have different physical and chemical properties.
For example:

(ii) General physical properties of geometrical isomer of but-2-ene
| (i) | Stability | trans cis |
| (ii) | Dipole moment | cis trans |
| (iii) | Boiling point | cis trans |
| (iv) | Melting point | trans cis |
(iii) Calculation of number of geometrical isomers
| Unsymmetrical | |
| Symmetrical |
(If is even) (If is odd) |
Where number of sites where is possible.
7. Optical isomerism:
Compounds having similar molecular and structural formulae but differing in the stereo chemical formula and the behaviour towards plane polarised light are called as optical isomers and this phenomenon is called as optical isomerism.
(i) Types of optical isomers:
(a) Optically active: dextrorotatory and laevorotatory
(b) Optically inactive: Meso
(ii) Condition: Molecule should be asymmetric or chiral i.e., symmetry element ( and ) should be absent.
(a) The carbon atom linked to four different groups is called the chiral carbon.
(iii) Fischer projection: An optical isomer can be represented by Fischer projection which is a planar representation of three-dimensional structure.
(a) Fischer projection representation of lactic acid:
(b) (-hydroxypropanoic acid)
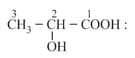
(c) 
(iv) Configuration of optical isomer:
(v) Determination of R/S configuration:
(a) Rule-: Assign the priority to the four groups attached to the chiral carbon according to the priority rule.
(b) Rule-: If lowest priority is bonded to a vertical line then moving.
is bonded to a vertical line then moving.
(c) 
(d) Rule-: If lowest priority is bonded to the horizontal line then moving.
is bonded to the horizontal line then moving.
(e) 
(vi) Determination of D/L system:
(a) Reference molecule is glyceraldehyde.
(b) It is used to assign configuration in carbohydrate, amino acid and similar compounds.
(c) Rule: Arrange parent carbon chain on the vertical line.
(d) Place the most oxidised carbon on the top or nearest to top.
(e) On highest numbered chiral carbon:
(f) If group on
(g) If group on
(h) 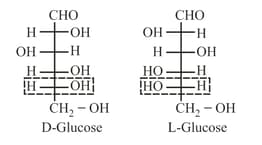
(vii) CIP Sequence rule: The following rules are followed for deciding the precedence order of the atoms or groups.
(a) Highest priority is assigned to the atoms of higher atomic number attached to asymmetric carbon atoms.
(b) In case of isotopes, isotopes having higher atomic mass are given priority.
(c) If the first atom of a group attached to an asymmetric carbon atom is same, then we consider the atomic number of 2nd atoms or subsequent atoms in a group.
(d) If there is a double bond or triple bond, both atoms are duplicated or triplicated.
(viii) Non-superimposable mirror images are called enantiomers which rotate the plane polarised light up to the same extent but in the opposite direction.
(ix) Diastereomers are stereoisomers which are not mirror images of each other. They have different physical and chemical properties.
(x) Meso compounds are those compounds whose molecules are superimposable on their mirror images despite the presence of asymmetric carbon atoms.
(xi) An equimolar mixture of the enantiomers ( and ) is called racemic mixture. The process of converting - or - form of an optically active compound into a racemic form is called racemisation.
(xii) The process by which mixture is separated into and forms with the help of chiral reagents or chiral catalysts are known as resolution.
(xiii) Compounds containing chiral carbon may or may not be optically active but show optical isomerism.
(xiv) For optical isomers, chiral carbon is not the necessary condition.
(xv) Calculation of number of optical isomers:
| The compound if contains | Optically active forms | Optically inactive forms(meso) |
| Unsymmetrical | Zero | |
| Symmetrical If even | ||
| Symmetrical If odd |
Where number of chiral carbons.
8. Conformational isomerism:
(i) The different arrangement of atoms in space that results from the carbon-carbon single bond free rotation by are called conformations or conformational isomers or rotational isomers and this phenomenon is called as conformational isomerism.
(ii) Newman projection: Here two carbon atoms forming the bonds are represented, one by circle and the other by centre of the circle. Circle represents rear side C and its centre represents front side carbon. The bonds of front carbon are depicted from the centre of the circle while bonds of the back carbon are drawn from the circumference of the circle.

(iii) Conformations of butane:
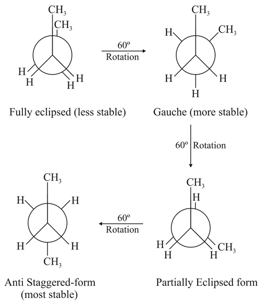
The order of stability of conformations of -butane.
Anti-staggeredGauchePartially eclipsedFully eclipsed.
Relative stability of various conformation of cyclohexane is Chair twist boat boat half chair
9. Fission of a Covalent Bond:
(i) Homolytic cleavage: In this cleavage, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms.
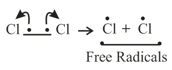
(ii) Heterolytic cleavage: In heterolytic cleavage the bond breaks in such a fashion that the shared pair of electrons remains with one of the fragments.
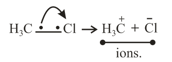
10. Electrophile:
Electrophiles are electron loving species having at least one vacant orbital in the valence shell.
(i) Charged electrophiles
(ii) Neutral Electrophiles: E.g.,
Note: Electrophiles are Lewis acids.
11. Nucleophile:
(i) Nucleophiles are nucleus loving, they are electron rich having at least one non-bonding pair of electrons in valence shell.
It can be of three types.
(a) Charged Nucleophiles -
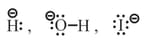 etc.
etc.
(b) Neutral Nucleophiles –
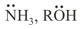
(c) Ambident Nucleophiles –
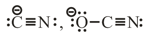
(both atoms are nucleophilic centre)
12. Electronic displacement effects in covalent bond:

(i) Inductive Effect:
(a) The permanent displacement of electrons in a bond towards the more electronegative element is called inductive effect.
(b) The effect provides polarity to the molecule. The property of electron withdrawal shown by an atom or group is its effect and that of donation is called effect.
(c) Electron donating or electron withdrawal is compared with respect to .
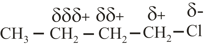
(d) As shown above the charge decreases from first position. Hence inductive effect decreases as the number of bonds increases.
Order of effect:
Order of effect:
Uses of Inductive Effect:
(i) Stability of ions:
Stability of ions can be explained by using the concept of inductive effect and hyperconjugation.
(a) Stability of Carbocation
e.g.,
(b) Stability of Carbanions
(groups with effect decrease the stability of anion)
(b) Acidic Properties:
It is possible to compare the acidic strength of various organic compounds using the inductive effect concept, for example, in carboxylic acid.
The strength of an acid depends on the ease with which it can ionize to give proton or on the stability of the conjugate bases formed, i.e., if the conjugate base formed is more stable, then the acid is more acidic.
| Name of Acid | Formula | Conjugate Base | |
|
Fluoroacetic acid (most acidic) |
(most stable) |
||
| Chloroacetic acid | |||
| Bromoacetic acid | |||
| Iodoacetic acid | |||
|
Acetic acid (least acidic) |
(least stable) |
Increasing stability of the conjugate base increases the acidity of acids.
Furthermore, the inductive effect in di and tri halogenated acids is still more marked with the result that they are progressively more acidic than the corresponding non-halogenated acids.
For example:
In general, dicarboxylic acids are stronger acids than monocarboxylic acids, since one of the group shows effect.
(ii) Hyperconjugation:
(a) It is also known as no-bond resonance.
(b) It involves the delocalisation of electrons of bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared orbital ( or orbital).
(c) Depending on the number of , various hyperconjugative structures are possible.
(d) Greater the number of hyperconjugative structures, higher would be the stability of the given system.
(e) It is also a permanent effect.
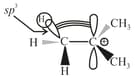
(f) Less effective overlap (responsible for hyperconjugative effect).
(g) Stability of substituted alkenes, alkyl carbocations, and free radicals can be explained based on hyper conjugation.
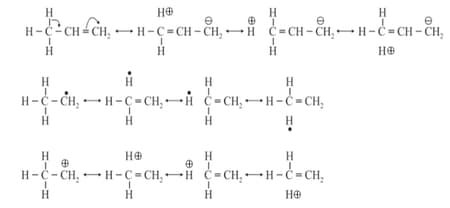
(iii) Electromeric Effect:
The temporary electron density charge in substrate in presence of attacking reagent facilitates the attack of attaching reagent is called electromeric effect.
(a) effect: When electron density piled on centre to which attacking reagent attacks, then the electromeric effect is effect, observed in the presence of electrophile. For example:
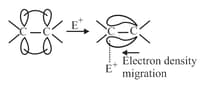
(b) effect: When electron density is removed from the centre to which attacking reagent attacks then electronic effect is effect, observed in the presence of nucleophiles. For example:
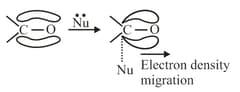
(iv) Mesomeric or Resonance Effect:
(a) The effect involves permanent delocalization of conjugated electrons in a conjugated system.
(b) Intermediate structures formed are called resonating structures.
(c) The structure that collectively represents all resonating structures is called the resonance hybrid.
(d) Resonating structures are imaginary and have no physical significance.
(e) The average structure i.e., resonance hybrid represents the actual molecule.
(f) Resonance is also known as -electron delocalisation, which is a permanent effect, it is a distance independent effect.
(g) Normally this effect is more powerful than the hyperconjugative effect.
(h) Resonating structures must be valid Lewis structures, they must have the same position of atomic nuclei, same number of paired and unpaired electrons and the part of molecule taking part in resonance must be planar.

(vi) Extent of contribution of various resonating structures:
(a) Resonating structures having maximum number of covalent bonds are more contributing.

(b) Non charge separated resonating structures are more contributing than charge separated resonating structures.
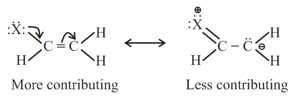
(c) Resonating structure which places negative charge on more electronegative atoms is more contributing as compared to resonating structure which places negative charge on less electronegative atom.
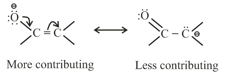
(d) The difference between energies of the most stable resonating structure and the resonance hybrid is called the resonance energy of the molecule.
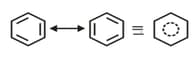
(e) Electron Donating:
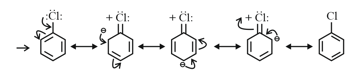
Mesomeric Effect ( Effect): Groups with effect releases -electron towards an unsaturated system. This effect makes certain positions in the molecule of high electron densities. This effect in chlorobenzene is shown as Effect
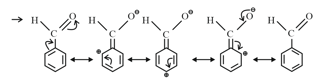
(f) Electron Withdrawing Mesomeric Effect Effect): This effect is observed when the displacement of -electrons from an unsaturated system is towards the atom or group. This effect in benzaldehyde is shown as Effect:
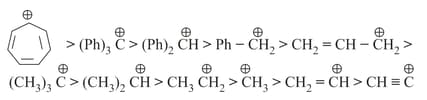
13. Relative stability order:
(i) Stability of carbocation
(ii) Stability of free radical:
(iii) Stability of Carbanion:
(iv) Acidic strength Stability of conjugate base
(a)
(b)
(c) 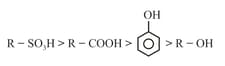
(d)
(e) 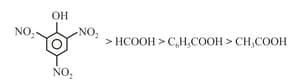
(f)
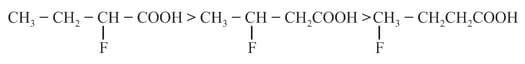
(g)

Phenol
(h)
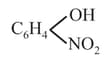
> Phenol
(i) 
(j)

benzoic acid

(k)
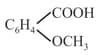
(l)

benzoic acid
(v) Basic strength of amine:
Basic strength
(a) In aqueous medium
(b) In gaseous medium
(vi) Order of electronic effect:
MesomericHyperconjugationInductive effect
(vii) Stability of alkene no. of –hydrogen:
Heat of hydrogenation
14. Detection and Estimation of elements in the organic compounds:
(i) Lassaigne's test for nitrogen:
(a) Lassiagne's extract is heated with solution in the presence of an alkali, the solution is cooled and then acidified with dilute .
(b) If a green or blue colouration is obtained, it confirms the presence of in the organic compound. The chemistry of the test is:
From organic compound
(Sod. Ferrocyanide)
This test is very delicate and is given by all compounds containing and . , etc. do not respond to this test since they do not contain carbon.
Formation of blood red colour indicates the presence of both and .
From organic compound Sod. thiocyanate or Sod. Sulphocyanide
(ii) Detection of sulphur:
If is present, during fusion with Na metal, is formed which may be tested as follows:
(a) With sodium nitroprusside violet colouration is produced:
(b) With lead acetate, black ppt. of is formed.
(iii) Detection of halogens:
When the organic compounds are fused with metal, the halogens combine with to form sodium halides. The presence of these halides is tested with solution.
(a) A white ppt. soluble in indicates chlorine.
(b) A pale-yellow ppt. partially soluble in ammonia indicates bromine.
(c) A yellow ppt. insoluble ammonia indicates iodine.
(d) If the organic compound also contains or the sodium extract is first boiled with dil. to decompose any cyanides or sulphides, otherwise these will form ppt. with solution.
(iv) Detection of phosphorus:
(a) Phosphorus is detected by fusing the organic compound with sodium peroxide, in which phosphorus is converted into sodium phosphate.
(b) The fused mass is extracted with and then boiled with conc. and then ammonium molybdate is added. Appearance of yellow ppt. or yellow colouration due to the formation of ammonium phosphomolybdate indicates the presence of phosphorus.
(v) Estimation of carbon and hydrogen:
Liebig's method: A known mass of the organic compound is heated strongly with excess of dry copper oxide in a current of dry air or oxygen (free from ) when carbon present in the organic compound compound is oxidised to and hydrogen to .
Percentage of carbon
Percentage of carbon
(vi) Estimation of nitrogen:
(a) Dumas method:
Small amounts of oxides of nitrogen
Oxides of nitrogen
Percentage of nitrogen
(b) Kjeldahl's method:
Organic compound
Percentage of nitrogen:
(vii) Estimation of halogen and Sulphur: (Carius method):
Percentage of chlorine
Percentage of bromine
Percentage of iodine
Percentage of sulphur
(viii) Estimation of phosphorus:
A known mass of the organic compound is heated with fuming in a Carius tube when phosphorus of the organic compound is oxidized to . Phosphoric acid thus formed is precipitated as magnesium ammonium phosphate by adding magnesia mixture (a solution containing and .)
Percentage of phosphorus:
(ix) Estimation of oxygen: A definite mass of an organic compound is decomposed by heating with gas. The mixture is then passed over red-hot coke when all oxygen is converted to CO. This mixture is then passed through when is oxidised to producing iodine. The of oxygen can be derived from the amount of , or produced.
Percentage of oxygen:
15. Purification methods:
(i) Distillation Techniques:
| Type | Conditions | Examples | |
| (A) | Simple distillation |
(i) When liquid sample has non volatile İmpurities (ii) When boiling point difference is or more. |
(i) Mixture of chloroform , and Aniline (ii) Mixture of Ether Toluene (iii) Hexane and Toluene |
| (B) |
Fractional distillation |
When difference is |
(i) Crude oil in petroleum industry (ii) Acetone and Methyl alcohol |
| (C) |
Distillation under reduced pressure (Vacuum distillation) |
When liquid boils at higher temperature and it may decompose before BP is attained. |
(i) Concentration of sugar juice (ii) Recovery of glycerol from spent lye. (iii) Glycerol |
| (D) | Steam distillation |
When the substance is immiscible with water and steam volatile.
|
(i) Aniline is separated from water (ii) Turpentine oil (iii) Nitro Benzene (iv) Bromobenzene (v) Naphthalene (vi) -Nitrophenol |
(ii) Other Methods of Purification of organic compounds:
(a) Crystallisation: Process of solidification of a pure substance from its dissolved state. This method is based upon differences in their solubility in the given solvent or in mixture of solvents.
(b) Sublimation: It is a process of conversion of a solid into gaseous state on heating without interchanging into liquid. The process is used for the separation of volatile solids, which sublime on heating from the non-volatile solids.
(c) Distillation: It is a process of conversion of a liquid into vapours by heating followed by condensation of vapours. The method is used for the purification of liquids which boil without decomposition and are present with non-volatile impurities.
(d) Fractional distillation: Process used to separate mixture of two or more miscible liquids having different boiling points. It is mainly used in distillation of petroleum, coal tar and crude alcohol.
(e) Distillation under reduced pressure: This process is used when the liquid tends to decompose near its boiling point. Under reduced pressure, the liquid will boil at a low temperature without decomposing.
(f) Steam distillation: Purification of a substance from non-volatile impurities provided the substance itself is volatile in steam and insoluble in water.
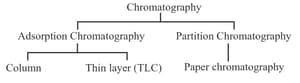
(g) Chromatography: Technique of separating the constituents of a mixture by the differential movement of individual components through the stationary phase under the influence of mobile phase. Two types of chromatography: