MEDIUM
Earn 100
List out the uses of sodium carbonate.
Important Questions on Elements of Group 1 and Group 2
EASY
Hydrogen peroxide is stored away from light because it can get decomposed when exposed to light as
MEDIUM
The reaction of aqueous with in acidic conditions gives:
HARD
Which of the following salt would give with hot and dil and also decolourises water?
EASY
The gas produced when metal is heated with aqueous is
EASY
In which of the following reactions, hydrogen peroxide acts as an oxidizing agent?
MEDIUM
Calcite crystals used in Nicol's prism are formed of
EASY
Choose the incorrect statement.
MEDIUM
Products and of the following reactions (I and II) are :
I.
II.
MEDIUM
Hydrogen peroxide oxidises in acidic medium, but reduces in alkaline medium. The other products formed are, respectively
MEDIUM
Hydrogen peroxide in its reaction with KIO4 and NH2OH respectively, is acting as a
EASY
Which among the following gas is bubbled through the brine solution during the preparation of sodium carbonate in Solvay's process?
EASY
The chemical nature of hydrogen peroxide is:
EASY
What is the product of the reaction of with
EASY
Identify and respectively in the following reactions
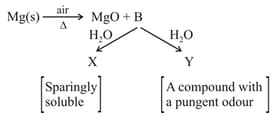
EASY
Sodium hydroxide is manufactured by
MEDIUM
The following reactions show the behaviour in and reactions as:
EASY
A hydrated solid X on heating initially gives a monohydrated compound Y. Y upon heating above leads to an anhydrous white powder Z. X and Z, respectively, are;
MEDIUM
When is added to an acidified solution
HARD
Number of water molecules in washing soda and soda ash respectively are :

