MEDIUM
Earn 100
List the characteristic physical properties of the gases.
Important Questions on States of Matter: Gaseous and Liquid States
EASY
Which of the following graphs represents Charle's law?
EASY
A spherical balloon of radius containing helium gas has a pressure of bar. At the same temperature, the pressure, of a spherical balloon of radius containing the same amount of gas will be bar.
EASY
of a gas at occupies a volume of . At what temperature will the volume be doubled, if pressure and amount of the gas remains constant?
EASY
For the given isotherms, which of the following is correct for
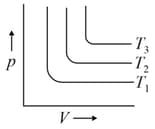
EASY
Modern vacuum pumps can evacuate a vessel down to a pressure of atm. at room temperature (300 K). Taking R = 8.3 JK-1 mole-1, 1 atm = 105 Pa and , the mean distance between molecules of gas in an evacuated vessel will be of the order of :
MEDIUM
The temperature to which the open vessel at is to be heated to expel of the air in it is (Assume (i) volume of the vessel remains constant and (ii) ideal behaviour for all gases present in air)
MEDIUM
The minimum pressure required to compress of a gas at bar to at is
EASY
An LPG cylinder contains gas at a pressure of at . The cylinder can withstand the pressure of . The room in which the cylinder is kept catches fire. The minimum temperature at which the bursting of cylinder will take place is __________ . (Nearest integer)
EASY
Equal masses of and methane have been taken in a container of volume at temperature in identical conditions. The ratio of the volumes of gases methane would be
MEDIUM
A mixture of gases and are taken in a closed vessel containing charcoal. The graph that represents the correct behaviour of pressure with time is:
EASY
For all gases, at any given pressure, the graph of volume vs temperature (in Celisius) is a straight line, This graph is called
MEDIUM
At a constant pressure P, the plot of volume (V) as a function of temperature (T) for 2 moles of an ideal gas gives a straight line with a slope . The value of P (in atm) is closest to
[Gas constant, ]
MEDIUM
A car tyre is filled with nitrogen gas at psi at . It will burst if pressure exceeds psi. The temperature in at which the car tyre will burst is (Rounded-off to the nearest integer)
MEDIUM
A certain sample of gas has a volume of at one atmosphere pressure and . What is the volume of gas at at same pressure ?
EASY
Slope of the plot between and at constant temperature is:
EASY
Which one of the following is the correct vs plot at constant temperature for an ideal gas ? ( and stand for pressure and volume of the gas respectively)
MEDIUM
Choose the correct option for graphical representation of Boyle's law, which shows a graph of pressure vs. volume of a gas at different temperatures:
EASY
Which of the following equations does NOT represent Charles’s law for a given mass of gas at constant pressure?
EASY
In a closed vessel, an ideal gas at is heated from , the final pressure of the gas will approximately be
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
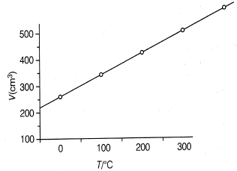
The volume of the gas at is larger than that at by a factor of

