EASY
AS and A Level
IMPORTANT
Earn 100
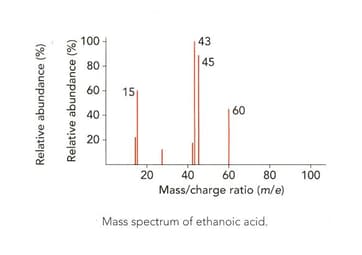
Look at the mass spectrum of ethanoic acid.
Identify the fragment with mass-to-charge ratio of .


Important Questions on Atoms, Molecules and Stoichiometry
EASY
AS and A Level
IMPORTANT
A hydrocarbon has molecular ion peak at mass-to-charge ratio of (relative abundance ) and an peak with a relative abundance of .
How many carbon atoms are in the hydrocarbon?
EASY
AS and A Level
IMPORTANT
List the ions responsible for the , and peaks in the mass spectrum of dibromomethane.
EASY
AS and A Level
IMPORTANT
What would be the mass-to-charge ratio and relative abundances of the major peaks with the highest charge-to mass ratios in the mass spectrum of chloroethane?
EASY
AS and A Level
IMPORTANT
How many peaks would you see beyond the molecular ion peak in -dibromoethane? What would be their mass-to-charge rations and abundances relative to the molecular ion? (Ignore peaks due to ).
EASY
AS and A Level
IMPORTANT
Use the value of sulphur is to calculate the amount of substance in moles in of sulphur atoms.
EASY
AS and A Level
IMPORTANT
Use the value of sulphur is to calculate the amount of substance in moles in of sulphur molecules .
EASY
AS and A Level
IMPORTANT
Use these values to calculate the amount of substance in moles in of anhydrous iron(III) nitrate, .
EASY
AS and A Level
IMPORTANT
Use the value of the Avogadro constant to calculate the total number of atoms in of chlorine atoms. ( value: ).
