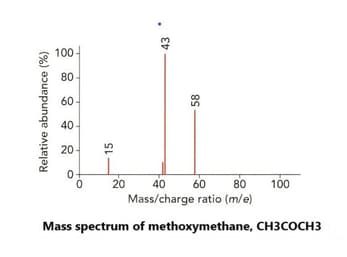
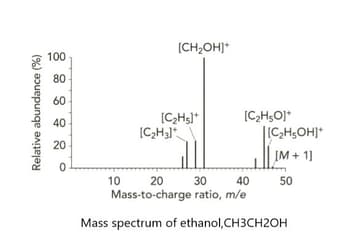
Look at the mass spectrum of ethanol, . A structural isomer of ethanol is methoxy methane, an ether with the formula .
Predict the mass-to-charge ratio of a fragment that would appear on the mass spectrum of methoxy methane but does not appear on methanol's mass spectrum.

Important Questions on Atoms, Molecules and Stoichiometry
Look at the mass spectrum of ethanol, . A structural isomer of ethanol is methoxy methane, an ether with the formula .
Predict and give the name of the fragment that would appear on the mass spectrum of methoxy methane but does not appear on methanol's mass spectrum.
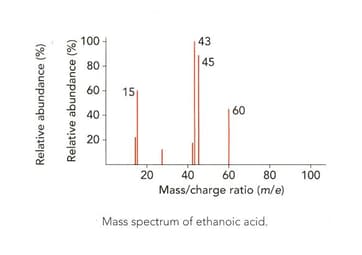
Look at the mass spectrum of ethanoic acid.
Identify the fragment with mass-to-charge ratio of .
A hydrocarbon has molecular ion peak at mass-to-charge ratio of (relative abundance ) and an peak with a relative abundance of .
How many carbon atoms are in the hydrocarbon?